Rationality for the Use of Neuromodulation in Disorders of Gut-Brain Interaction (Functional Gastrointestinal Disorders)
DOI:
https://doi.org/10.22516/25007440.997Keywords:
Functional Gastrointestinal Disorders, neuromodulation, antidepressantsAbstract
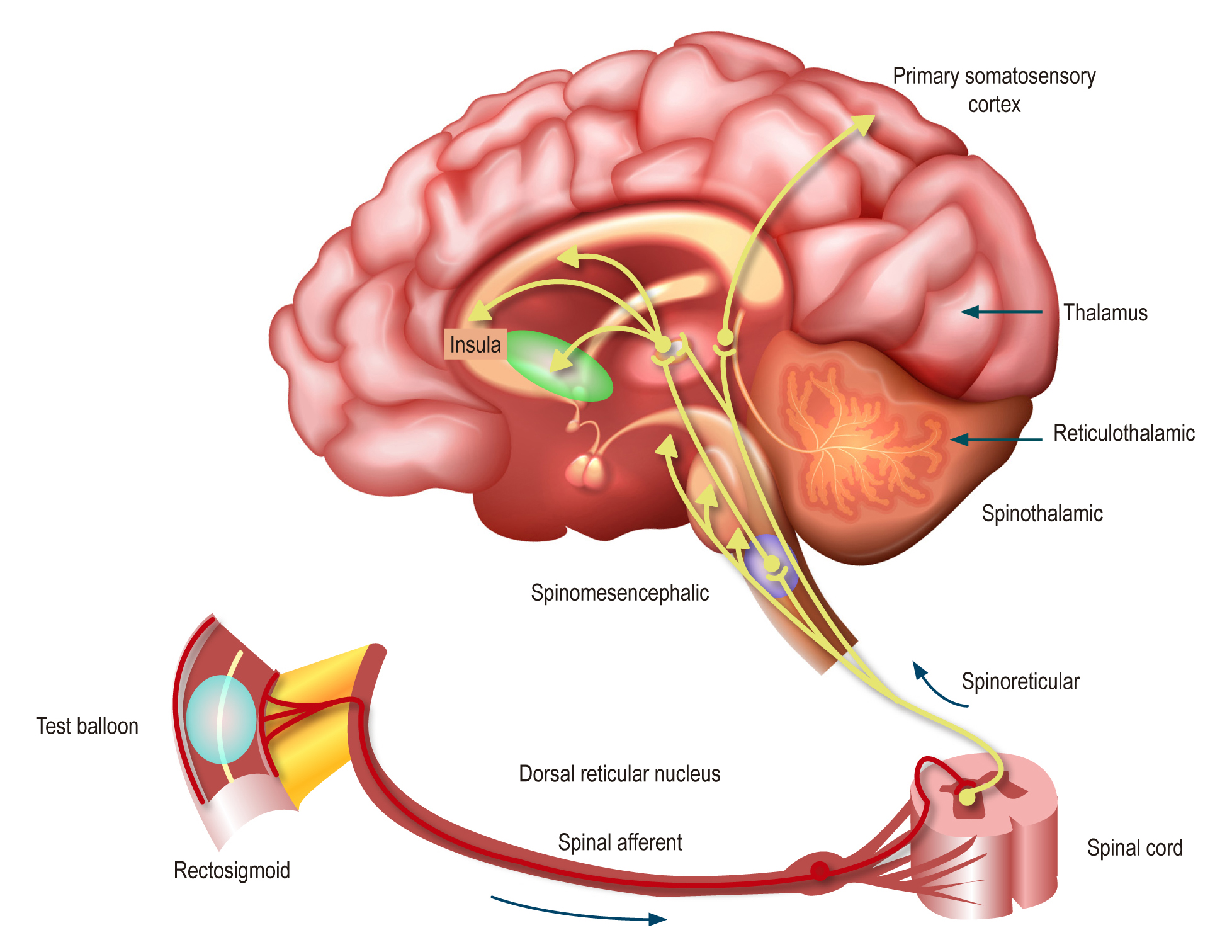
Within the broad range of therapeutic options for managing functional gastrointestinal disorders, recently redefined as Disorders of Gut-Brain Interaction (DGBI) by the Rome Foundation in the Rome IV criteria, certain medications with antidepressant, anxiolytic, or antipsychotic effects are commonly employed. These drugs, now referred to as neuromodulators by the Rome Foundation, target the neurogastroenterological dysfunction associated with these disorders. Consequently, their clinical utility as psychiatric medications can now be leveraged to benefit patients with DGBI.
This narrative review aims to provide an updated and specific overview of the indications for neuromodulators in the primary DGBI. The first section of this review focuses on the rationale and justification for their use.
Downloads
References
Drossman DA, Hasler WL. Rome IV-Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology. 2016;150(6):1257-61. https://doi.org/10.1053/j.gastro.2016.03.035
Drossman DA, Tack J, Ford AC, Szigethy E, Törnblom H, van Oudenhove L. Neuromodulators for Functional Gastrointestinal Disorders (Disorders of Gut-Brain Interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018;154(4):1140-1171.e1. https://doi.org/10.1053/j.gastro.2017.11.279
Vanner SJ, Greenwood-Van Meerveld B, Mawe GM, Shea-Donohue T, Verdu EF, Wood J, et al. Fundamentals of Neurogastroenterology: Basic Science. Gastroenterology. 2016;150(6):1280-91. https://doi.org/10.1053/j.gastro.2016.02.018
Abdullah N, Defaye M, Altier C. Neural control of gut homeostasis. Am J Physiol Gastrointest Liver Physiol. 2020;319(6):G718-G732. https://doi.org/10.1152/ajpgi.00293.2020
Jin P, Jan LY, Jan YN. Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu Rev Neurosci. 2020;43:207-29. https://doi.org/10.1146/annurev-neuro-070918-050509
Kiela PR, Ghishan FK. Physiology of Intestinal Absorption and Secretion. Best Pract Res Clin Gastroenterol. 2016;30(2):145-59. https://doi.org/10.1016/j.bpg.2016.02.007
Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17(6):338-51. https://doi.org/10.1038/s41575-020-0271-2
Foong D, Zhou J, Zarrouk A, Ho V, O’Connor MD. Understanding the Biology of Human Interstitial Cells of Cajal in Gastrointestinal Motility. Int J Mol Sci. 2020;21(12):1-18. https://doi.org/10.3390/ijms21124540
Greenwood-Van Meerveld B, Johnson AC. Stress-Induced Chronic Visceral Pain of Gastrointestinal Origin. Frontiers in Systems Neuroscience. 2017;11:86. https://doi.org/10.3389/fnsys.2017.00086
Elam JS, Glasser MF, Harms MP, Sotiropoulos SN, Andersson JLR, Burgess GC, et al. The Human Connectome Project: A retrospective. Neuroimage. 2021;244:118543. https://doi.org/10.1016/j.neuroimage.2021.118543
Kano M, Grinsvall C, Ran Q, Dupont P, Morishita J, Muratsubaki T, et al. Resting state functional connectivity of the pain matrix and default mode network in irritable bowel syndrome: a graph theoretical analysis. Scientific Reports. 2020;10(1):11015. https://doi.org/10.1038/s41598-020-67048-9
Turkiewicz J, Bhatt RR, Wang H, Vora P, Krause B, Sauk JS, et al. Altered brain structural connectivity in patients with longstanding gut inflammation is correlated with psychological symptoms and disease duration. Neuroimage Clin. 2021;30:102613. https://doi.org/10.1016/j.nicl.2021.102613
Vergnolle N. Postinflammatory visceral sensitivity and pain mechanisms. Neurogastroenterol Motil. 2008;20 (Suppl 1):73-80. https://doi.org/10.1111/j.1365-2982.2008.01110.x
Piche T. Tight junctions and IBS--the link between epithelial permeability, low-grade inflammation, and symptom generation? Neurogastroenterol Motil. 2014;26(3):296-302. https://doi.org/10.1111/nmo.12315
Boyer J, Saint-Paul MC, Dadone B, Patouraux S, Vivinus MH, Ouvrier D, et al. Inflammatory cell distribution in colon mucosa as a new tool for diagnosis of irritable bowel syndrome: A promising pilot study. Neurogastroenterol Motil. 2018;30(1). https://doi.org/10.1111/nmo.13223
Hong JY, Labus JS, Jiang Z, Ashe-Mcnalley C, Dinov I, Gupta A, et al. Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. PLoS One. 2014;9(1):e84564. https://doi.org/10.1371/journal.pone.0084564
Mills EP, Keay KA, Henderson LA. Brainstem Pain-Modulation Circuitry and Its Plasticity in Neuropathic Pain: Insights From Human Brain Imaging Investigations. Frontiers in pain research (Lausanne, Switzerland). 2021;2:705345. https://doi.org/10.3389/fpain.2021.705345
van Oudenhove L, Crowell MD, Drossman DA, Halpert AD, Keefer L, Lackner JM, et al. Biopsychosocial Aspects of Functional Gastrointestinal Disorders. Gastroenterology. 2016;150(6):1355-1367.e2. https://doi.org/10.1053/j.gastro.2016.02.027
Drossman DA. Abuse, trauma, and GI illness: is there a link? Am J Gastroenterol. 2011;106(1):14-25. https://doi.org/10.1038/ajg.2010.453
Bouchoucha M, Hejnar M, Devroede G, Babba T, Bon C, Benamouzig R. Anxiety and depression as markers of multiplicity of sites of functional gastrointestinal disorders: a gender issue? Clin Res Hepatol Gastroenterol. 2013;37(4):422-30. https://doi.org/10.1016/j.clinre.2012.10.011
Dimsdale JE, Creed F, Escobar J, Sharpe M, Wulsin L, Barsky A, et al. Somatic symptom disorder: an important change in DSM. J Psychosom Res. 2013;75(3):223-8. https://doi.org/10.1016/j.jpsychores.2013.06.033
Radu M, Moldovan R, Pintea S, Băban A, Dumitrașcu D. Predictors of outcome in cognitive and behavioural interventions for irritable bowel syndrome. A meta-analysis. J Gastrointestin Liver Dis. 2018;27(3):257-63. https://doi.org/10.15403/jgld.2014.1121.273.bab
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Revista colombiana de Gastroenterología

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.


| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |














