Stomach Cancer and Postendoscopy Colorectal Cancer: Parallel Lives
DOI:
https://doi.org/10.22516/25007440.1145Keywords:
Stomach cancer, postendoscopy colorectal cancer, colorectal cancer, postcolonoscopy colorectal cancer, endoscopy, colonoscopy, survivalAbstract
Background and objectives: The rates of (interval) stomach cancer (SC) or postendoscopy (PECRC) or postcolonoscopy (PCCRC) colorectal cancer (CRC) have been little studied in our setting. Data from overseas studies reported PECRC and PCCRC rates of 7–26%. We aim to determine and compare local PECRC and PCCRC rates and characteristics.
Patients and methods: With data from three quaternary-care cancer centers, we ambispectively identified patients diagnosed with SC and CRC between 2012 and 2021, in whom a history of endoscopies or colonoscopies in the previous three years was investigated. Cancers diagnosed between 6 and 36 months after an endoscopic study reported as normal were defined as interval cancers. This study compares the clinical, endoscopic, and survival characteristics of both PECRC and PCCRC cohorts.
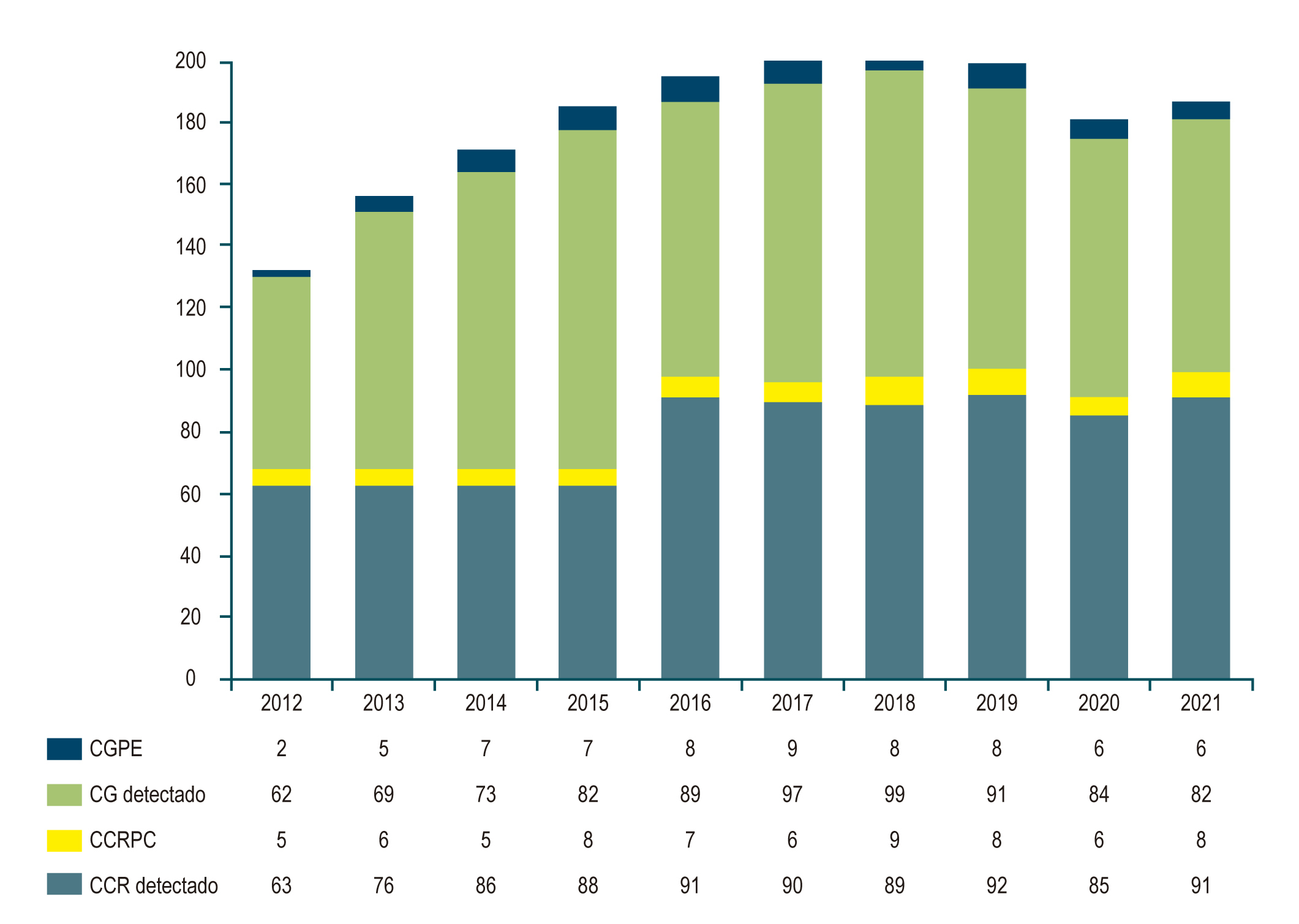
Results: Of 828 patients diagnosed with SC, 66 had PECRC (rate: 7.3%), while in 919 patients with CRC, 68 had PCCRC (rate: 6.9%). There were no significant differences in age or sex, although males predominated (2:1) in the PECRC (0.09). The finding of premalignant lesions was similar in both groups (p = 0.260). The anatomical location was shown to be more proximal (right colon) in the PCCRC than in the PECRC (cardia/fundus) (p = 0.002). Gastric neoplasms were more poorly differentiated (58%) than colon neoplasms (26%) (p = 0.001). There were no differences in early cancers, but tumor status was more advanced in PECRC (p < 0.01). The Kaplan-Meier showed a worse survival for PCCRC than for detected CRC, with no differences in SC and PECRC, suggesting poor survival.
Conclusions: The rate of interval cancers is 7.3% and 6.9%, and differences were found between PECRC and PCCRC, proximal locations of the lesions, degree of differentiation, tumor status, and poor survival for the PCCRC. Establishing measures to achieve the World Endoscopy Organization’s goal of <5% is necessary.
Downloads
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin.;72(1):7-33. https://doi.org/10.3322/caac.21708
Piñeros M, Laversanne M, Barrios E, Cancela MC, de Vries E, Pardo C, et al. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg Health Am. 2022;13:None. https://doi.org/10.1016/j.lana.2022.100294
Song M. Global epidemiology and prevention of colorectal cancer. Lancet Gastroenterol Hepatol. 2022;7(7):588-90. https://doi.org/10.1016/S2468-1253(22)00089-9
Morgan E, Arnold M, Camargo MC, Gini A, Kunzmann AT, Matsuda T, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study. EClinicalMedicine. 2022;47:101404. https://doi.org/10.1016/j.eclinm.2022.101404
Luu XQ, Lee K, Jun JK, Suh M, Jung KW, Choi KS. Effect of gastric cancer screening on long-term survival of gastric cancer patients: results of Korean national cancer screening program. J Gastroenterol. 2022;57(7):464-75. https://doi.org/10.1007/s00535-022-01878-4
Ibáñez-Sanz G, Sanz-Pamplona R, Garcia M, On Behalf Of The Msic-Sc Research Group. Future Prospects of Colorectal Cancer Screening: Characterizing Interval Cancers. Cancers (Basel). 2021;13(6):1328. https://doi.org/10.3390/cancers13061328
Xia JY, Aadam AA. Advances in screening and detection of gastric cancer. J Surg Oncol. 2022;125(7):1104-9. https://doi.org/10.1002/jso.26844
Teixeira C, Martins C, Dantas E, Trabulo D, Mangualde J, Freire R, et al. Interval colorectal cancer after colonoscopy. Rev Gastroenterol Mex. 2019;84(3):284-9. https://doi.org/10.1016/j.rgmxen.2018.04.008
Telford JJ, Enns RA. Endoscopic missed rates of upper gastrointestinal cancers: parallels with colonoscopy. Am J Gastroenterol. 2010;105(6):1298-300. https://doi.org/10.1038/ajg.2009.739
Castaño-Llano R, Piñeres A, Jaramillo R, Molina S, Aristizábal F, Puerta JE. Interval gastric cancer: A call to attentiveness and action. Rev Gastroenterol Mex (Engl Ed). 2023;88(2):91-99. https://doi.org/10.1016/j.rgmxen.2022.05.015
Januszewicz W, Kaminski MF. Quality indicators in diagnostic upper gastrointestinal endoscopy. Therap Adv Gastroenterol. 2020;13:1756284820916693. https://doi.org/10.1177/1756284820916693
Sanduleanu S, le Clercq CMC, Dekker E, Meijer GA, Rabeneck L, Rutter MD, et al. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64(8):1257-67. https://doi.org/10.1136/gutjnl-2014-307992
Pabby A, Schoen RE, Weissfeld JL, Burt R, Kikendall JW, Lance P, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61(3):385-91. https://doi.org/10.1016/S0016-5107(04)02765-8
Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109(9):1375-89. https://doi.org/10.1038/ajg.2014.171
Park MS, Yoon JY, Chung HS, Lee H, Park JC, Shin SK, et al. Clinicopathologic Characteristics of Interval Gastric Cancer in Korea. Gut Liver. 2015;9(2):167-73. https://doi.org/10.5009/gnl13425
Plutarco. Vidas paralelas. Buenos Aires: Losada; 2010.
Beck M, Bringeland EA, Qvigstad G, Fossmark R. Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016-A Population-Based Study. Cancers (Basel). 2021;13(22):5628. https://doi.org/10.3390/cancers13225628
Ren J, Kirkness CS, Kim M, Asche CV, Puli S. Long-term risk of colorectal cancer by gender after positive colonoscopy: population-based cohort study. Curr Med Res Opin. 2016;32(8):1367-74. https://doi.org/10.1080/03007995.2016.1174840
Kolata G. Colonoscopies Miss Many Cancers, Study Finds. The New York Times [Internet]. 2008 [citado el 29 de julio de 2022]. Disponible en: https://www.nytimes.com/2008/12/16/health/16cancer.html
Amin A, Gilmour H, Graham L, Paterson-Brown S, Terrace J, Crofts TJ. Gastric adenocarcinoma missed at endoscopy. J R Coll Surg Edinb. 2002;47(5):681-4.
Pimenta-Melo AR, Monteiro-Soares M, Libânio D, Dinis-Ribeiro M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-Analysis. Eur J Gastroenterol Hepatol. 2016;28(9):1041-9. https://doi.org/10.1097/MEG.0000000000000657
Kim KO, Huh KC, Hong SP, Kim WH, Yoon H, Kim SW, et al. Frequency and characteristics of interval colorectal cancer in actual clinical practice: A KASID multicenter study. Gut Liver. 2018;12(5):537-43. https://doi.org/10.5009/gnl17485
Laurent E, Hussain H, Calvin Poon TK, Ayantunde AA. The Incidence, Distribution and Clinicopathology of Missed Colorectal Cancer After Diagnostic Colonoscopy. Turk J Gastroenterol. 2021;32(11):988-94. https://doi.org/10.5152/tjg.2021.20500
Teixeira C, Martins C, Dantas E, Trabulo D, Mangualde J, Freire R, et al. Interval colorectal cancer after colonoscopy. Rev Gastroenterol Méx (English Edition). 2019;84(3):284-9. https://doi.org/10.1016/j.rgmxen.2018.04.008
Forsberg A, Hammar U, Ekbom A, Hultcrantz R. Post-colonoscopy colorectal cancers in Sweden: Room for quality improvement. Eur J Gastroenterol Hepatol. 2017;29(7):855-60. https://doi.org/10.1097/MEG.0000000000000884
Cheung KS, Chen L, Seto WK, Leung WK. Epidemiology, characteristics, and survival of post-colonoscopy colorectal cancer in Asia: A population-based study. J Gastroenterol Hepatol (Australia). 2019;34(9):1545-53. https://doi.org/10.1111/jgh.14674
Burr NE, Derbyshire E, Taylor J, Whalley S, Subramanian V, Finan PJ, et al. Variation in post-colonoscopy colorectal cancer across colonoscopy providers in English National Health Service: Population based cohort study. BMJ. 2019:367:l6090. https://doi.org/10.1136/bmj.l6090
Macken E, Van Dongen S, De Brabander I, Francque S, Driessen A, Van Hal G. Post-colonoscopy colorectal cancer in Belgium: characteristics and influencing factors. Endosc Int Open. 2019;7(5):E717-E727. https://doi.org/10.1055/a-0751-2660
Pedersen L, Valori R, Bernstein I, Lindorff-Larsen K, Green C, Torp-Pedersen C. Risk of post-colonoscopy colorectal cancer in Denmark: Time trends and comparison with Sweden and the English National Health Service. Endoscopy. 2019;51(8):733-41. https://doi.org/10.1055/a-0919-4803
Strum WB, Boland CR. Interval Colorectal Cancer 2006-2015: Novel Observations. Dig Dis Sci. 2021;66(3):855-60. https://doi.org/10.1007/s10620-020-06242-1
Laish I, Mizrahi J, Naftali T, Konikoff FM. Diabetes Mellitus and Age are Risk Factors of Interval Colon Cancer: A Case-Control Study. Digestive Diseases. 2019;37(4):291-6. https://doi.org/10.1159/000496740
Morris EJA, Rutter MD, Finan PJ, Thomas JD, Valori R. Post-colonoscopy colorectal cancer (PCCRC) rates vary considerably depending on the method used to calculate them: a retrospective observational population-based study of PCCRC in the English National Health Service. Gut. 2015;64(8):1248-56. https://doi.org/10.1136/gutjnl-2014-308362
Rutter MD, Beintaris I, Valori R, Chiu HM, Corley DA, Cuatrecasas M, et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology. 2018;155(3):909-925.e3. https://doi.org/10.1053/j.gastro.2018.05.038
Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of New or Missed Colorectal Cancers After Colonoscopy and Their Risk Factors: A Population-Based Analysis. Gastroenterology. 2007;132(1):96-102. https://doi.org/10.1053/j.gastro.2006.10.027
le Clercq CMC, Bouwens MWE, Rondagh EJA, Bakker CM, Keulen ETP, de Ridder RJ, et al. Postcolonoscopy colorectal cancers are preventable: A population-based study. Gut. 2014;63(6):957-63. https://doi.org/10.1136/gutjnl-2013-304880
Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: A population-based study. Am J Gastroenterol. 2010;105(12):2588-96. https://doi.org/10.1038/ajg.2010.390
Cooper GS, Xu F, Schluchter MD, Koroukian SM, Barnholtz Sloan JS. Diverticulosis and the Risk of Interval Colorectal Cancer. Dig Dis Sci. 2014;59(11):2765. https://doi.org/10.1007/s10620-014-3246-8
Cubiella J. Closing the gap for post-colonoscopy colorectal cancer. Lancet Gastroenterol Hepatol. 2022;7(8):694-5. https://doi.org/10.1016/S2468-1253(22)00128-5
Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality Indicators for Colonoscopy and the Risk of Interval Cancer. NEJM. 2010;362(19):1795-803. https://doi.org/10.1056/NEJMoa0907667
Singh R, Chiam KH, Leiria F, Pu LZCT, Choi KC, Militz M. Chromoendoscopy: role in modern endoscopic imaging. Transl Gastroenterol Hepatol. 2020;5:39. https://doi.org/10.21037/tgh.2019.12.06
Wallace MB, Sharma P, Bhandari P, East J, Antonelli G, Lorenzetti R, et al. Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia. Gastroenterology. 2022;163(1):295-304.e5. https://doi.org/10.1053/j.gastro.2022.03.007
Arribas Anta J, Dinis-Ribeiro M. Early gastric cancer and Artificial Intelligence: Is it time for population screening? Best Pract Res Clin Gastroenterol. 2021;52-53:101710. https://doi.org/10.1016/j.bpg.2020.101710
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Revista colombiana de Gastroenterología

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Aquellos autores/as que tengan publicaciones con esta revista, aceptan los términos siguientes:
Los autores/as ceden sus derechos de autor y garantizarán a la revista el derecho de primera publicación de su obra, el cuál estará simultáneamente sujeto a la Licencia de reconocimiento de Creative Commons que permite a terceros compartir la obra siempre que se indique su autor y su primera publicación en esta revista.
Los contenidos están protegidos bajo una licencia de Creative Commons Reconocimiento-NoComercial-SinObraDerivada 4.0 Internacional.


| Article metrics | |
|---|---|
| Abstract views | |
| Galley vies | |
| PDF Views | |
| HTML views | |
| Other views | |














